- India
- International
Britain approves AstraZeneca/Oxford Covid-19 vaccine
AstraZeneca said the authorisation was for a two dose regime, and that the vaccine had been approved for use for emergency supply. Britain has ordered 100 million doses of the vaccine.
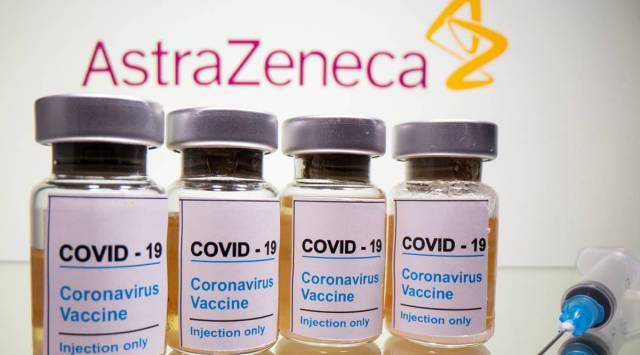 Vials with a sticker reading, "COVID-19 / Coronavirus vaccine / Injection only" and a medical syringe are seen in front of a displayed AstraZeneca logo in this illustration. (Reuters)
Vials with a sticker reading, "COVID-19 / Coronavirus vaccine / Injection only" and a medical syringe are seen in front of a displayed AstraZeneca logo in this illustration. (Reuters)Written by Benjamin Mueller and Rebecca Robbins
Britain became the first country Wednesday to give emergency authorization to the coronavirus vaccine developed by AstraZeneca and the University of Oxford, opening a path for a cheap and easy-to-store shot that much of the world will rely on to help end the pandemic.
In a bold decision to accelerate vaccinations, a British government advisory body directed clinicians to give as many people as possible their first dose of a coronavirus vaccine, without reserving supplies for planned second doses.
Instead of administering the two shots within a month, clinicians will wait as long as 12 weeks to give people their second doses, the government said, a decision that applies to the Oxford-AstraZeneca vaccine as well as the Pfizer-BioNTech shot that Britain authorized early this month.
With the vaccination rollout moving slowly in both Britain and the United States, British officials were heeding calls to delay the second doses as a way of giving more people the partial protection of a single dose. Matt Hancock, the health secretary, said people would start receiving the AstraZeneca vaccine early next week.

For Britain, where hospitals are overwhelmed by a deluge of cases of a new, more contagious variant of the virus, the decision by its drug regulator offered some hope of a reprieve. The health service is preparing to soon vaccinate 1 million people per week at makeshift sites in soccer stadiums and racecourses.
The Oxford-AstraZeneca shot is poised to become the world’s dominant form of inoculation. At $3 to $4 a dose, it is a fraction of the cost of some other vaccines. And it can be shipped and stored at normal refrigeration temperatures for six months, rather than in the ultracold freezers required by rival vaccines from Pfizer-BioNTech and Moderna, making it easier to administer to people in poorer and harder-to-reach parts of the world.
When given in two, full-strength doses, AstraZeneca’s vaccine showed 62% efficacy in clinical trials — considerably lower than the roughly 95% efficacy achieved by Pfizer and Moderna’s shots. For reasons scientists don’t yet understand, AstraZeneca’s vaccine showed 90% efficacy in a smaller group of volunteers who were given a half-strength initial dose.
In announcing the authorization Wednesday, Britain’s Department of Health did not immediately outline which dosing regimen had been authorized, or whether clinicians would be given leeway to choose between the two.
Beyond the dosing questions, Britain’s health service must also figure out how to persuade people to take a vaccine that appears less effective than other available shots, but that nevertheless could hasten the end of a pandemic that has been killing hundreds of people each day in Britain and thousands more around the world.
The authorization relied on data from late-stage clinical trials in Britain and Brazil. India’s drug regulator is also expected to decide soon whether to authorize the vaccine, which is being manufactured there by a local vaccine producer, the Serum Institute.
A decision is further off in the United States, where the Food and Drug Administration is waiting for data from a separate clinical trial. The study was halted in September and delayed for nearly seven weeks — much longer than in other countries — as regulators investigated whether an illness in a participant in Britain was related to the vaccine. U.S. regulators ultimately allowed the trial to proceed.
In recent days, the Oxford scientists who developed the vaccine have waded into a debate on both sides of the Atlantic about whether to delay the planned second doses of this and other vaccines as a way of giving more people the partial protection of a single dose. Andrew Pollard, the director of the Oxford Vaccine Group, said in a radio interview Monday that “it makes a lot of sense to get started with as many people as possible” by delaying the second dose.
AstraZeneca has set more ambitious manufacturing targets than other vaccine makers, saying that it expects to be able to make up to 3 billion doses next year. At two doses per person, that would be enough to inoculate nearly 1 in 5 people worldwide. The company has pledged to make it available at cost around the world until at least July 2021 and in poorer countries in perpetuity.
But the firm has also been dogged by communication blunders that have damaged its relationship with U.S. regulators and raised doubts about whether the vaccine will stand up to intense public and scientific scrutiny. Those blunders have set back the vaccine’s timeline in the United States, where key FDA officials were stunned to have learned of the pause in its clinical trials in September not from AstraZeneca, but from the news media.
Having ordered 100 million doses, 40 million of which are supposed to be available by March, Britain has made the AstraZeneca shot the linchpin of its vaccination strategy. Since authorizing Pfizer’s vaccine Dec. 2, Britain has used it to vaccinate hundreds of thousands of people. But the country has struggled to administer it beyond hospitals and doctor’s offices, leaving some of its highest-priority recipients, like nursing home residents, still vulnerable.
A small number of volunteers in the clinical trial in Britain received their first dose at half strength because of a measurement problem. Oxford had hired an outside manufacturer to produce the vaccine for the trial. But when researchers received a sample of the vaccine, they found its strength to be twice as high as what the manufacturer had found using a different measurement technique. Not knowing which measurement to trust, the researchers decided to cut the dose in half to ensure that volunteers wouldn’t get double the dose originally intended. Later, the Oxford researchers confirmed that their reading was too high, and they switched back to the originally planned dose for the second shot.
In the smaller group of 2,741 people who received the half-strength first dose or a meningococcal vaccine as a control, the vaccine was found to have 90% efficacy. None of those participants were over the age of 55, however, making it difficult to know if those results would hold in older people.
AstraZeneca and Oxford scientists have said that they do not know why the half-strength initial dose was so much more effective. But they have voiced confidence in their results, particularly the finding that no one who received the vaccine in the clinical trials developed severe COVID-19 or was hospitalized.
“We think we have figured out the winning formula and how to get efficacy that, after two doses, is up there with everybody else,” Pascal Soriot, AstraZeneca’s chief executive, told The Times of London in an interview published Saturday. The company has released no evidence of efficacy rates as high as Pfizer or Moderna’s. “I can’t tell you more because we will publish at some point,” Soriot told The Times.
Oxford scientists earlier this month published interim findings from the vaccine’s clinical trials in The Lancet. Forthcoming final results from those trials are not expected to be significantly different from the interim data, as is typical in clinical research.
Must Read
Apr 27: Latest News
- 01
- 02
- 03
- 04
- 05